Two projects are currently going on in the group: one is about the design and synthesis of proteases inhibitors and the other one is based on the asymmetric synthesis and derivatization of oxiranic compounds:
Design and synthesis of cysteine proteases inhibitors
We have synthesized four families of potent parasitic cysteine proteases inhibitors belonging to the family of papain. Some cysteine proteases of the papain family are therapeutic targets for the search of drugs against infectious tropical diseases such as Chagas disease (cruzain), sleeping sickness (Rhodesian) or malaria (falcipains). Currently we are also working in the developing of inhibitors of SARS-CoV-2 cysteine proteases.
The compounds developed in our group possess a general chemical structure, it is a dipeptidic framework that is effectively recognized by the residues of the active center of the enzyme, and a warhead that reacts with the thiolate of the cysteine:
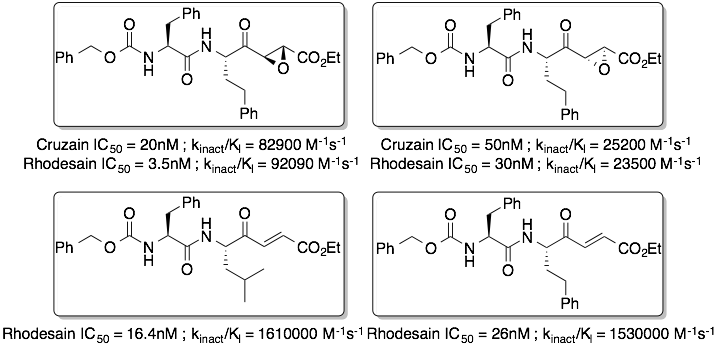
Dipeptidyl epoxyesters and ketoenoates are potent irreversible inhibitors of cysteine proteases cruzain and rhodesain:
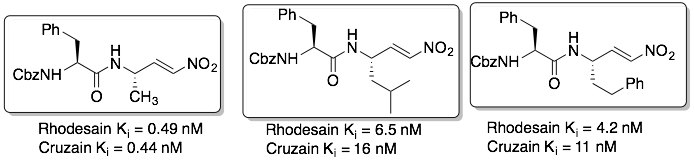
We have also synthesized dipeptidyl nitroalkenes which are reversible inhibitors:
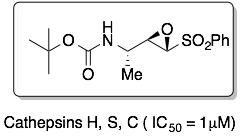
Inhibitors with an epoxysulfone as an electrophilic trap are active against human cathepsins inhibitors:
Asymmetric synthesis and derivatization of oxiranic compounds
We have performed highly diastereoselective epoxidation reactions of electron-deficient alkenes having an oxygenated stereocenter:
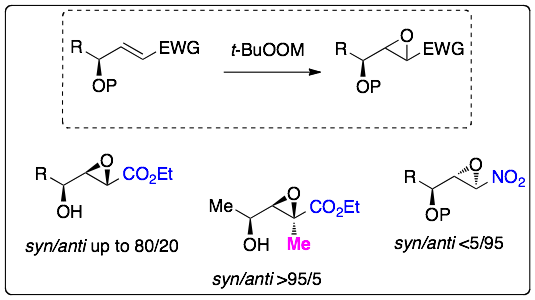
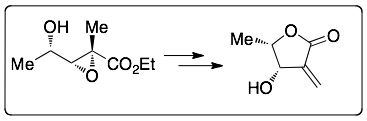
The obtained epoxides have applied to the syntheses of interesting compounds, such as an alpha-methylene-beta-hydroxy-gamma-butyrolactone which is a natural product:
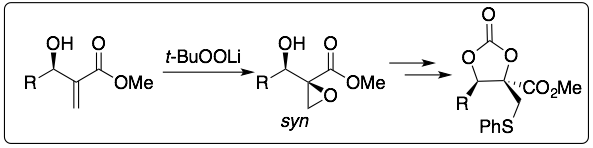
A similar study was performed over Morita-Baylis Hillman adducts:
We reported an efficient ring opening of 3,4-epoxyesters with alcohols to produce 4-alkoxy-3-hydroxyesters and their isomerization into 4-ketoesters using boron trifluoride as catalyst. Both transformations are simple and efficient methods for the synthesis of above named synthetically useful compounds.
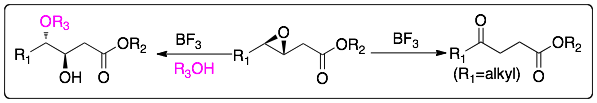
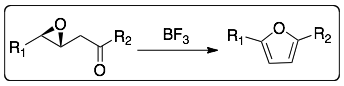
If 3,4-epoxyketones are treated with boron trifluoride then furans are prepared.
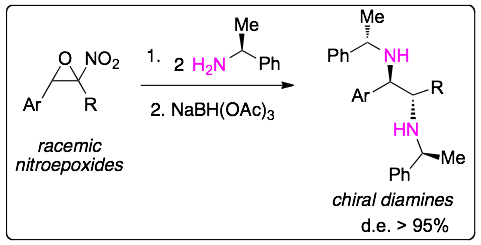
We have reported an enantioselective synthesis of chiral vicinal diamines starting from nitroepoxides using a chiral amine and a reductive agent. The process is a dynamic kinetic asymmetric transformation (DYKAT):
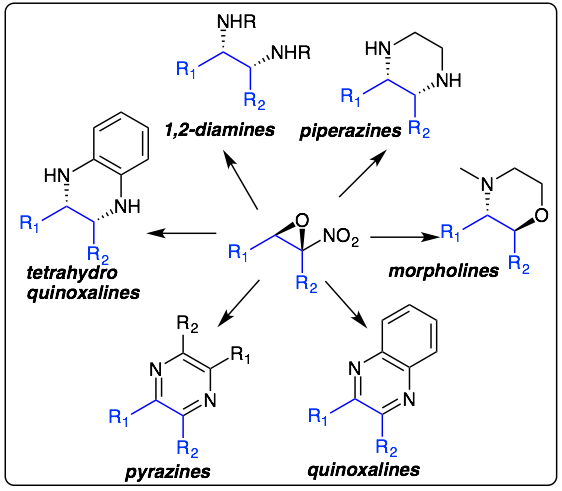
We reported nitroepoxides are easily transformed into 1,4-diamino heterocycles such as quinoxalines and pyrazines by treatment with 1,2-benzenediamines and ammonia respectively. Additionally, related saturated heterocycles, such as piperazines and tetrahydroquinoxalines can be accessed by treatment with 1,2-diamines and a reducing agent. Recently we have reported the transformation of nitroepoxides into morpholines and benzoxazines:
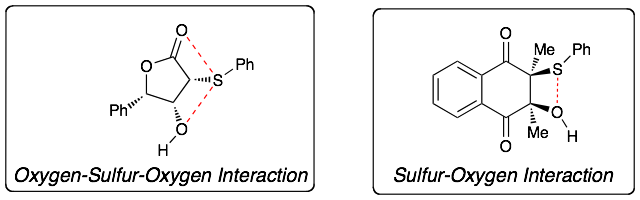
We have also reported that sulfur-oxygen interactions are the main driving force during isomerization of cyclic alpha-sulfurated aldols: